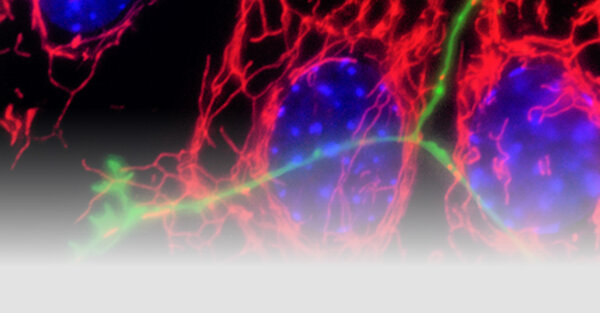
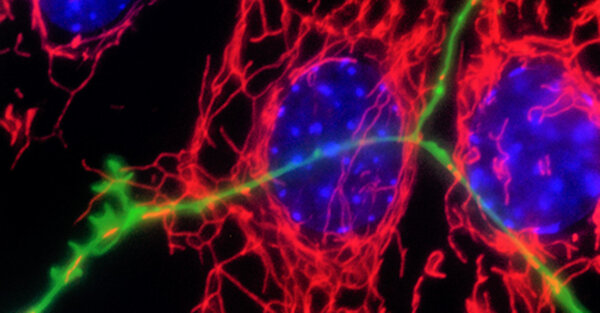
Neurodegenerative disorders are associated in a proportion of cases with genetic risk and gene mutations. However, the vast majority of Parkinson’s disease cases are sporadic and the disease is altogether heterogeneous in symptoms and pathology. The Fitzgerald group aims to understand the molecular mechanisms underlying neurodegeneration using genetic forms of the disease as an entry point and bridge our findings using patient-derived biofluids to model sporadic cases and predict the diagnosis and/or progression of the disease.
Mitochondria are crucial organelles that produce energy and perform many other functions needed for central metabolism and cell signalling. As neurons use a lot of energy, they rely on tight control for bioenergetic efficiency and mitochondrial quality by reducing oxidative burden. Mitochondrial dysfunction is a phenomenon that traverses all neurodegenerative diseases and may explain the selective vulnerability of certain brain cells. This is especially relevant in Parkinson’s disease, where the dopaminergic neurons degenerate because the mitochondria are a major source of oxidative stress and the location of dopamine degradation.
A large body of work has depicted the role of mitochondrial dysfunction in certain genetic forms of Parkinson’s disease, yet the exact mechanisms underlying sporadic forms of the disease are less defined.
We utilize several different model cell systems with a focus on patient-derived cells. In close collaboration with the Hertie Institute biobank and the Neurology Clinics, we are currently working on primary human fibroblasts derived from patients and healthy individuals, induced pluripotent stem cells (iPSCs), small molecule neuronal progenitor cells (smNPCs), mature dopaminergic neurons and peripheral blood mononuclear cells (PBMCs) from patients and healthy individuals.
We perform mostly functional work, focusing on biology, biochemistry and physiology and have established several specialized techniques for monitoring mitochondrial health. We make use of our established techniques including but not limited to CRISPR gene-editing, protein biochemistry, imaging and flow cytometry methods.

PINK1 in Parkinson’s disease
Molecular mechanisms associated with PINK1-Parkinson’s disease
Karan Sharma
Mutations in mitochondrial PTEN-induced putative kinase 1 (PINK1) are a common cause of recessively inherited Parkinson’s disease. However, the mechanisms by which PINK1 can lead to the death of dopaminergic neurons at the substantia nigra remain elusive. To study the disease, we primarily utilize human iPSC-derived dopaminergic neurons cultured in vitro. In addition to observing “the well-defined” canonical mitophagy impairment upon mitochondrial membrane depolarization, we observe metabolic differences in PINK1 loss of function dopaminergic neurons, related to but not limited to the mitochondria. By diving deeper through collaborative multi-omic approaches, i.e., phospho/proteomics and lipidomics, and using these datasets as a reference entry point, we have identified 1. increased cholesterol biosynthesis leading to a build-up of cholesterol in PINK1 knock-out dopaminergic neurons and 2. PINK1 influences dopamine metabolism via the regulation of Tyrosine Hydroxylase, the rate-limiting enzyme for dopamine synthesis. In these projects, we aim to investigate the molecular mechanisms through which PINK1 influences these altered pathways in dopaminergic neurons in the hope of incorporating therapeutics that could in future benefit patients affected by Parkinson’s disease.
This study is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Research Training Group MOMbrane 654651/GRK2364. MOMbrane aims to investigate the various functions of the mitochondrial outer membrane (MOM) as well as its structure, regulation and biogenesis. The MOMbrane includes ten PhD students in different institutes in Tübingen, each focusing on different aspects of the MOM. The project is carried out in collaboration with partner labs at the Weizmann Institute in Rehovot (Israel). Link to the RTG MOMbrane website. https://www.mombrane.de/
The role of the mitochondrial outer membrane protein Miro1 in Parkinson’s disease
Miro1 function in dopaminergic neurons
Lisa Schwarz (Alumni)
We introduced disease-associated (RHOT1 R272Q) and functional (RHOT1 S156A, and K572R) Miro1/RHOT1 mutations in human iPSCs using gene editing. Healthy and edited iPSCs can then be differentiated into other cell types including neurons (Schwarz, Casadei and Fitzgerald. 2021). We studied the function of Miro1 using these domain and pathway specific mutations by assessing mitochondrial turnover, mitophagy metabolism and calcium handling (Schwarz and Fitzgerald, 2022, Schwarz et al. 2022).
This study is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Research Training Group MOMbrane 654651/GRK2364. https://www.mombrane.de/
Miro1 retention at depolarized mitochondria in Parkinson’s disease
Layla Drwesh
Miro1 is a mitochondrial outer membrane protein with atypical two GTPase domains surrounding two central EF-hands (calcium binding domains). Its primary function is to facilitate mitochondrial movement along the microtubules by anchoring mitochondria via adaptor proteins TRAK1/2 to molecular motors. Mitochondrial trafficking is necessary for proper mitochondrial distribution for neuronal health. In addition to mitochondrial motility, Miro1 is heavily implicated in mitochondrial quality control since its depletion is a well-known marker of classical CCCP induced mitophagy. Furthermore, Miro1 interacts with PINK1 (Weihofen et al., 2009) and Parkin (Birsa et al.,2014, Klosowiak et al., 2016) making it an important player in PINK1-Parkin mediated mitochondrial quality control. Miro1 was tied to PD biology following the discovery that LRRK2 promotes Miro1 removal by forming a complex with it (Hsieh et al., 2016). In the same study it was shown for the first time using human fibroblasts from patients that Miro1 degradation is impaired in sporadic PD leading to delayed mitophagy. However, it is still unclear why Miro1 retention following mitochondrial depolarization occurs in some PD cells and not others. To address this gap and better understand the frequency of Miro1 retention in PD, this study aims to measure Miro1's response to mitochondrial depolarization in an independent cohort of PD patients and healthy controls from Tübingen. We utilize available biomaterials such as fibroblasts and blood for this investigation. By validating Miro1 as a robust cellular hallmark for PD, this research will contribute to therapeutic interventions targeting the Miro1-defected phenotype in PD patients.
This project is funded by The Michael J Fox Foundation. MJFF-021967.
BEST - Biomarker evaluation to support clinical translation in schizophrenia
Maria Zarani & Richard Wüst
Psychosis represents a group of diseases signified by enormous heterogeneity. As a consequence, diagnosis, prognosis, and treatment decisions for individual patients are pretty challenging. Within the project, we shall optimize biomarker signatures of patients with high risk for schizophrenia and with defined psychotic disease. This will be achieved by integration of multimodal patient data and subsequent validation of biomarker signatures with the help of patient-derived neurons.
The project is funded through the BMBF (FRN: 01EK2101A) and is carried out in collaboration with four partners; LMU München, ZI Mannheim, NMI Reutlingen and the Hertie Institut for Clinical Brain Research/University Hospital for Psychiatry Tübingen (HIH/UKPP). The validation of mitochondrial readouts will be performed by the HIH/UKPP.
The role of the endosomal-lysosomal system in atypical Parkinson’s disease/Corticobasal syndrome
Katharina Stegen (Alumni), Lisa Schwarz (Alumni) and Julia Fitzgerald
Dysfunction of the endosomal-lysosomal system is a phenomenon that traverses many neurodegenerative diseases including Alzheimer’s diseases, Parkinson’s disease and other rarer neurodegenerative diseases such as progressive supranuclear palsy (PSP) and corticobasal syndrome/ degeneration (CBS/CBD). The endosomal lysosomal system is formed and driven by changes in pH and therefore the ion exchangers that reside in the membranous compartments of the endosomal lumen are critical for correct pH regulation. These exchangers are crucial and major defects often cause severe mental retardation. We are investigating changes to the endolysosomal system related to the sodium hydrogen exchanger 6 (NHE6) that could contribute towards susceptibility to develop neurodegenerative diseases in later life.
This project was funded by the Fortüne program of the Medical Faculty, University of Tübingen. This project is carried out in collaboration with Rejko Krüger (LCSB, Luxembourg and Luxembourg Institute of Health, Luxembourg).
Mitochondrial DNA as a biomarker for Parkinson’s disease
Julia Fitzgerald, Gerrit Machetanz, Anne Grünewald (LCSB, Université du Luxembourg)
Sporadic forms of Parkinson’s disease are the most prevalent but they are difficult to model in the laboratory because there is no known gene mutation that can be introduced or corrected to generate isogenic controls. Statistically, large cohorts are needed to compare healthy individuals with sporadic Parkinson’s disease patients. This poses a problem because culturing patient-derived cell lines in parallel for such large cohorts requires specialist robotics, it is invasive for patients and is also expensive. To overcome this, we are collecting biofluids from a large cohort of Parkinson’s disease patients including sporadic and familial cases where mitochondrial proteins are affected. We will look for changes in mitochondria in these cells to try to identify subgroups of patients that may in future benefit from medicines that target mitochondrial dysfunction.
This project is funded by The Michael J Fox Foundation. MJFF-15744, MJFF-021147

+49 (0)7071-
29-81971

2025
Drwesh L, Arena G, Merk DJ, Ferrante D, Gorgogietas V, Gasser T, Grünewald A, May P, Brockmann K, Krüger R, Wüst R, Gloeckner CJ, Fitzgerald JC. Methodological validation of Miro1 retention as a candidate Parkinson's disease biomarker. NPJ Parkinsons Dis. 2025 Sep 15;11(1):270. doi: 10.1038/s41531-025-01115-8. PMID: 40954161
Fitzgerald JC, Bremm A. A structural switch reveals how to disarm USP30 and unlock mitophagy. Nat Struct Mol Biol. 2025 Sep;32(9):1586-1588. doi: 10.1038/s41594-025-01640-3. PMID: 40715444
Cavarischia-Rega C, Sharma K, Fitzgerald JC, Macek B. Proteomic insights into the biology of dopaminergic neurons. Front Mol Neurosci. 2025 Jul 30;18:1642519. doi: 10.3389/fnmol.2025.1642519. PMID: 40808910
Tsiami F, Drwesh L, Surender S, Fitzgerald J, Schittenhelm J, Picketts DJ, Segal RA, Tabatabai G, Merk DJ. SMARCA5 is required for the development of granule cell neuron precursors and Sonic Hedgehog Medulloblastoma growth. Sci Rep. 2025 Jul 18;15(1):26091. doi: 10.1038/s41598-025-11857-3. PMID: 40681754
Fitzgerald JC, Sun Y, Reinecke F, Bauer E, Garaschuk O, Kahle PJ, Pfeiffer F. Interactions of Oligodendrocyte Precursor Cells and Dopaminergic Neurons in the Mouse Substantia Nigra. J Neurochem. 2025 Jan;169(1):e16298. doi: 10.1111/jnc.16298. PMID: 39871627
2024
Cavarischia-Rega C, Sharma K, Fitzgerald JC, Macek B. Proteome Dynamics in iPSC-Derived Human Dopaminergic Neurons. Mol Cell Proteomics. 2024 Oct;23(10):100838. doi: 10.1016/j.mcpro.2024.100838. Epub 2024 Sep 7. PMID: 39251023
Sharma K, Kishore A, Lechado-Terradas A, Passannanti R, Raimondi F, Sturm M, Sreelatha AAK, Puthenveedu DK, Sarma G, Casadei N, Krüger R, Gasser T, Kahle P, Riess O, Fitzgerald JC, Sharma M. A Novel PINK1 p.F385S Loss-of-Function Mutation in an Indian Family with Parkinson's Disease. Mov Disord. 2024 Apr 8. doi: 10.1002/mds.29792. Epub ahead of print. PMID: 38586902.
2023
Breitmeyer R, Vogel S, Heider J, Hartmann SM, Wüst R, Keller AL, Binner A, Fitzgerald JC, Fallgatter AJ, Volkmer H. Regulation of synaptic connectivity in schizophrenia spectrum by mutual neuron-microglia interaction. Commun Biol. 2023 Apr 29;6(1):472. doi: 10.1038/s42003-023-04852-9. PMID: 37117634; PMCID: PMC10147621.
Schumacher L, Slimani R, Zizmare L, Ehlers J, Kleine Borgmann F, Fitzgerald JC, Fallier-Becker P, Beckmann A, Grißmer A, Meier C, El-Ayoubi A, Devraj K, Mittelbronn M, Trautwein C, Naumann U. TGF-Beta Modulates the Integrity of the Blood Brain Barrier In Vitro, and Is Associated with Metabolic Alterations in Pericytes. Biomedicines. 2023 Jan 14;11(1):214. doi: 10.3390/biomedicines11010214. PMID: 36672722; PMCID: PMC9855966.
2022
Lechado-Terradas A, Schepers S, Zittlau K, Sharma K, Ok O, Fitzgerald JC, Westermann GB, Macek B and Kahle PJ. (2022). Parkin-dependent mitophagy occurs via proteasome-dependent steps sequentially targeting separate mitochondrial sub-compartments for autophagy. AUTOPHAGY REPORTS, VOL. 1, NO. 1, 576–602. doi.org/10.1080/27694127.2022.2143214.
Schwarz L, Sharma K, Dodi LD, Rieder LS, Fallier-Becker P, Casadei N, Fitzgerald JC. Miro1 R272Q disrupts mitochondrial calcium handling and neurotransmitter uptake in dopaminergic neurons. Front Mol Neurosci. 2022 Dec 2;15:966209. doi: 10.3389/fnmol.2022.966209. PMID: 36533136; PMCID: PMC9757607.
Schmidt S, Luecken MD, Trümbach D, Hembach S, Niedermeier KM, Wenck N, Pflügler K, Stautner C, Böttcher A, Lickert H, Ramirez-Suastegui C, Ahmad R, Ziller MJ, Fitzgerald JC, Ruf V, van de Berg WDJ, Jonker AJ, Gasser T, Winner B, Winkler J, Vogt Weisenhorn DM, Giesert F, Theis FJ & Wurst W. Primary cilia and SHH signaling impairments in human and mouse models of Parkinson’s disease. /Nat Commun/ 2022; 13, 4819 PMID: 35974013
Arena G, Sharma K, Agyeah G, Krüger R, Grünewald A, Fitzgerald JC. Neurodegeneration and Neuroinflammation in Parkinson's Disease: a Self-Sustained Loop /Curr Neurol Neurosci Rep/ 2022; June 8:1–14 PMID: 35674870
Harmuth T, Weber JJ, Zimmer AJ, Sowa AS, Schmidt J, Fitzgerald JC, Schöls L, Riess O, Hübener-Schmid J. Mitochondrial Dysfunction in Spinocerebellar Ataxia Type 3 Is Linked to VDAC1 Deubiquitination /Int. J. Mol. Sci/ 2022; 23, 5933 PMID: 35682609
Schwarz L and Fitzgerald JC. Steady-State Levels of Miro1 Linked to Phosphorylation at Serine 156 and Mitochondrial Respiration in Dopaminergic Neurons /Cells/ 2022; 11, no. 8: 1269. PMID:35455950
Kakade P, Ojha H, Raimi OG, Kakade P, Ojha H, Raimi OG, Shaw A, Waddell AD, Ault JR, Burel S, Brockmann K, Kumar A, Ahangar MS, Krysztofinska EM, Macartney T, Bayliss R, Fitzgerald JC, Muqit MMK. Mapping of a N-terminal α-helix domain required for human PINK1 stabilization, Serine228 autophosphorylation and activation in cells. /Open Biol./ 2022;12(1):210264 PMID: 35042401
2021
Schwarz L, Casadei N and Fitzgerald JC. Generation of R272Q, S156A and K572R RHOT1/Miro1 point mutations in iPSCs from a healthy individual using FACS-assisted CRISPR/Cas9 genome editing. /Stem Cell Res./ 2021 25(55), 102469. PMID: 34359002
Brown SJ, Boussaad I, Jazaro J, Fitzgerald JC, Antony P, Keatinge M, Blechman, J, Schwamborn J, Krüger R, Placzek M, Bandmann O. PINK1 deficiency impairs adult neurogenesis of dopaminergic neurons. /Sci Rep/ 2021; 11, 6617. PMID: 33758225
Körner A, Bernard A, Fitzgerald JC, Alarcon-Barrerer JC, Kostidis S, Kaussen T, Giera M & Mirakaj V. Sema7A is crucial for resolution of severe inflammation. /PNAS/ 2021; 118, e2017527118. PMID: 33637648
2020
Bus C, Zizmare L, Feldkaemper M, Geisler S, Zarani M, Schaedler A, Klose F, Admard, J, Mageean CJ, Arena J, Fallier-Becker P, Ugun-Klusek A, Maruszczak K, Kapolou K, Schmid B, Rapaport D, Ueffing M, Casadei N, Krüger R, Gasser T, Vogt-Weisenhorn D, Kahle PJ, Trautwein C, Gloeckner CJ and Fitzgerald JC. Human Dopaminergic Neurons Lacking PINK1 Exhibit Disrupted Dopamine Metabolism Related to Vitamin B6 Co-Factors./iScience/ 2020; 23, 12, 101797. PMID: 33299968
Stock R, Jeckel P, Kraushaar U, Wüst R, Fallgatter A, Volkmer H. The potential of induced pluripotent stem cells for discriminating neurodevelopmental disorders. Stem Cells Transl Med. 2021 Jan;10(1):50-56. doi: 10.1002/sctm.20-0206. Epub 2020 Aug 31. PMID: 32864861; PMCID: PMC7780807.
Stock R, Vogel S, Mau-Holzmann UA, Kriebel M, Wüst R, Fallgatter AJ, Volkmer H. Generation and characterization of human induced pluripotent stem cells lines from four patients diagnosed with schizophrenia and one healthy control. Stem Cell Res. 2020 Oct;48:101961. doi: 10.1016/j.scr.2020.101961. Epub 2020 Aug 27. PMID: 32911325.
2019
Hertlein V, Flores-Romero H, Das KK, Fischer S, Heunemann M, Calleja-Felipe M, Knafo S, Hipp K, Harter K, Fitzgerald JC, García-Sáez AJ. MERLIN: a novel BRET-based proximity biosensor for studying mitochondria-ER contact sites. /Life Sci Alliance/ 2019; Dec 9;3(1). PMID: 31818884
Grossmann D, Berenguer-Escuder C, Bellet M, Scheibner D, Bohler J, Massart F, Rapaport D, Skupin A, Fouquier d'Hérouël A, Sharma M, Ghelfi J, Raković A, Lichtner P, Antony P, Glaab E, May P, Dimmer K, Fitzgerald JC, Grünewald A, Krüger R. Mutations in RHOT1 Disrupt Endoplasmic Reticulum-Mitochondria Contact Sites Interfering with Calcium Homeostasis and Mitochondrial Dynamics in Parkinson's Disease. /Antioxid Redox Signal/ 2019; Dec 1;31(16):1213-1234. PMID: 31303019
2018
Ugun-Klusek A, Theodosi TS, Fitzgerald JC, Burté F, Ufer C, Boocock D, Yu-Wai-Man P, Bedford L, Billett EE. Monoamineoxidase-A promotes protective autophagy in human SH-SY5Y neuroblastoma cells through Bcl-2 phosphorylation. /Redox Biol./2018; 20,167-181.PMID: 30336354
Jores T, Lawatscheck J, Beke V, Franz-Wachtel M, Yunoki K, Fitzgerald JC, Macek B, Endo T, Kalbacher H, Buchner J, and Rapaport D. Cytosolic Hsp70 and Hsp40 chaperones enable the biogenesis of mitochondrial β-barrel proteins. /J. Cell. Biol./2018; Sep 3;217(9):3091-3108. PMID: 29930205
Fitzgerald JC, Zimprich A, Bobbili DR, May P, Sharma M, Krüger R. Reply: No evidence for rare TRAP1 mutations influencing the risk of idiopathic Parkinson’s disease. /Brain/ 2018; 141 (3):17. PMID: 29373630 * corresponding author
Sofi S, Fitzgerald JC, Jähn D, Dumoulin B, Stehling S, Kuhn H, Ufer C. Functional characterization of naturally occurring genetic variations of the human Guanine-rich RNA sequence binding factor 1 (GRSF1). /Biochim. Biophys. Acta/. 2018;1862(4):866-876. PMID: 29366917
Marrone L, Bus C, Schöndorf D, Fitzgerald JC, Kübler M, et al. Generation of iPSCs carrying a common LRRK2 risk allele for in vitro modeling of idiopathic Parkinson's disease. /PLOS One/2018; 13(3). PMID: 29513666
Rotermund C, Machetanz G, Fitzgerald JC. The Therapeutic Potential of Metformin in Neurodegenerative Diseases. /Frontiers in Endocrinology/ 2018; 19;9:400. PMID:
- Welcome to our new MSc graduate student Vignesh Anikode-Kasiviswanathan (Graduate Training Centre of Neuroscience, Tübingen) who is working on the characterization of SLC9A6/NHE6 in corticobasal syndrome.
- We welcome Loris Bandirali, a visiting PhD student from The University of Pavia.
- For more MOMbrane news and announcements please see www.mombrane.de
- At the moment, we are busy training students and interns and have already agreed on all student projects until 2026. Therefore, we have no further opportunities for Post-docs, PhD, MSc, BSc projects or internships.













